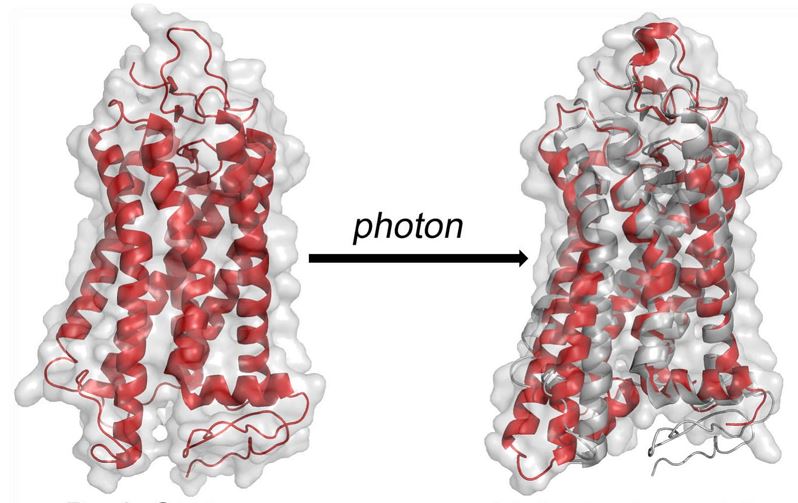
The advent of X-ray free-electron lasers (XFELs) opens up entirely new horizons in chemistry, biology, and physics. The XFEL pulses have a peak brilliance that is 10^9 stronger than any synchrotron X-ray radiation. Before the introduction of XFELs, the investigation of time-dependent change in crystals was restricted to Laue crystallography, which needs reversible photoreactions to acquire many light-dark diffraction patterns. The use of third-generation synchrotron sources requires larger microcrystals that can take months to grow, restrict conformation changes of proteins, and moreover radiation damage can occur. Our team is currently investigating the activation of rhodopsin by photons in small nanocrystals using the short intense X‐ray pulses of the free-electron laser at the Linac Coherent Light Source (LCLS) at SLAC National Accelerator Laboratory. Using pump‐probe technique activation of rhodopsin is studied in the sub‐picosecond to microsecond range. This allows us to probe the ultra-fast structural changes occur in the protein during very early stages of rhodopsin interaction with the photons. Extending these studies to the millisecond time scale where transition from inactive meta I to active meta II state, which activates the effector G-protein, transducin. Our ultimate goal is to produce a molecular movie of the GPCR activation process as a prototype for the Rhodopsin Family of G-protein–coupled receptors (GPCRs). Current projects involve preparing small nano/microcrystals of rhodopsin for pump-probe femtosecond X-ray studies. Time-resolved wide-angle X-ray scattering (WAXS) of rhodopsin in detergent micelles probes the light-induced ultra-fast conformational changes of the protein within a few picoseconds after photons interact with the retinal chromophore. These lightning-fast structural changes in the protein are termed as “protein quake”. We are also exploring how the X-ray free electron laser (XFEL) technology can be further improved to investigate other G-protein–coupled receptors (GPCRs) using rhodopsin as a prototype.
Small Angle X-ray scattering of rhodopsin with artificial membranes
The role of membrane-lipids is crucial in membrane proteins like in rhodopsin. In this study, our group is working on developing new method for spontaneously reconstituting bovine rhodopsin in artificial membrane using small-angle x-ray techniques and UV-visible spectroscopy.
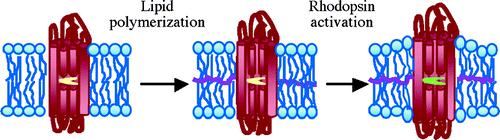
The advantage of this new method include: (1) No post-reconstitution reaction is needed to polymerize the membrane; (2) There is no chemical bonding between individual amphiphiles in block copolymer membranes. As such, the moduli of block copolymer membranes can be tuned from the most lipid-like to the least lipid-like by selecting polymer chains w/ different flexibility; (3) Unlike lipid bilayers, the thickness of block copolymer membrane has a wide range of tunability, and hydrophobic matching with MPs can be achieved without altering the curvature of the membranes; (4) The curvature, surface crowding, and surface chemistry of block copolymer membranes can be tuned by chemical design. The ability to functionally reconstitute bovine rhodopsin in rationally designed block copolymer membranes may have positive impact on harnessing other GPCRs for applications such as sensors and drug screening
Furthermore, how the membrane lipids affect water movement (whether there is influx or efflux or water) in rhodopsin recombined in the membrane lipids will further be studied.
Investigate photoactivation dynamics of rhodopsin in resolved time scales using SFX, and TR-SWAXS
This project involves an international team of scientists in which I continue to play a key role. Serial femtosecond crystallography (SFX) and time-resolved Small and Wide Angle- X-ray scattering (SWAXS) experiments are conducted at LCLS in Stanford University.
The Co-PIs for this project are Prof. Michael F. Brown, University of Arizona, Prof. Petra Fromme, and Prof. Richard Kirian, Arizona State University, along with Prof. Thomas, Grant, University at Buffalo, NY In this project, we study the protein structural changes occur during rhodopsin activation.
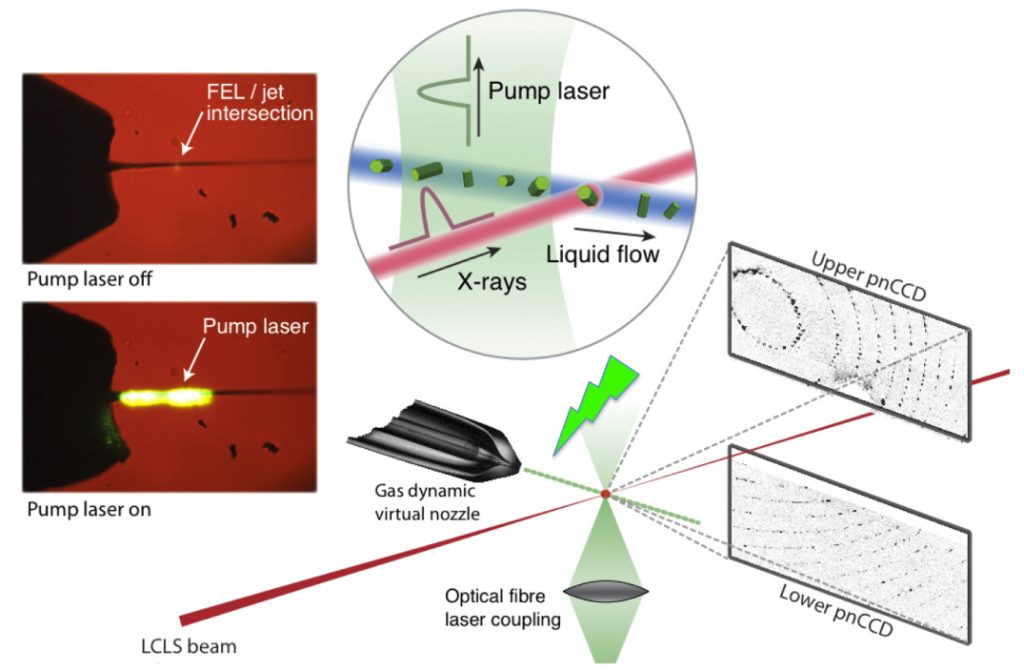
The figure on the left summarizes the processes involved in SFX and TR-WAXS imaging of the protein in highly resolved time scales using the free electron laser at LCLS. Here we introduce rhodopsin crystals (for SFX) or detergent solubilized rhodopsin (for TR-WAXS) using a liquid jet to the high flux free electron laser (FEL) beam. Due to the very high flux of the FEL, the rhodopsin crystals are destroyed instantaneously. However, using the cutting-edge technology we are able to collect diffraction patterns at very fast time scales. Photoactivation of the rhodopsin using a pump-probe laser at controlled time-periods allows us to monitor the structural changes of the protein in highly time-resolved manner. The TR-WAXS experiments conducted at LCLS in Spring 2014 revealed photon triggered protein dynamics occur in rhodopsin in picosecond time scales.
Study changes in rhodopsin shape using SANS and SAXS
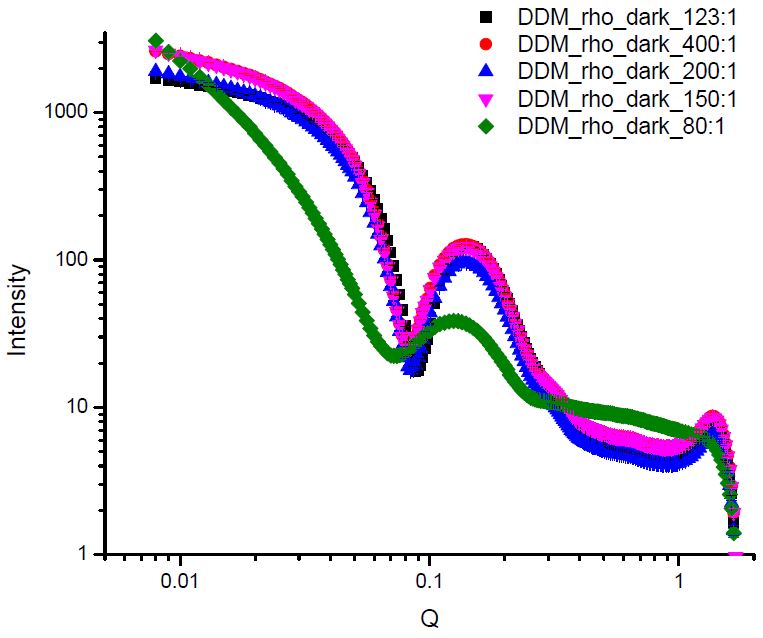
This project is a collaboration of the Brown group, the group of Prof. Xiang-Qiang Chu (Dept. of Physics) at Wayne State University, and Oak Ridge National Laboratory (ORNL). Here we study rhodopsin in different detergents that stabilize different intermediates upon photoactivation via SANS. My previous experiments at Spallation Neutron Source (SNS) and High Flux Isotope Reactor (HFIR) at ORNL revealed significant structural differences between the rhodopsin solubilized in detergents favor meta-I rhodopsin, and detergents that favor active meta II intermediate. Additional SAXS experiments conducted at Brookhaven National Laboratory (BNL) studied the effect of the detergent type and amount on the shape of rhodopsin.