How are retinal neurons damaged by diabetes?
Diabetic retinopathy is a common complication of diabetes involving vascular problems in the eye. However, recent studies in diabetic humans and animal models have shown that neural retinal activity changes cause decreases in vision that occur long before diabetic vascular growth. We are investigating this on the single cell level in the retina. We have shown specific dysfunction in light-evoked retinal signaling from amacrine cells to bipolar cells due to changes in calcium handling. We have also shown over-excitation of ganglion cells in both dark and light adapted conditions and compromised dopamine signaling. We are currently focusing on defining mechanisms of calcium and dopamine dysregulation in the diabetic retina.
Check out the podcast interview, article and radio interview about the Eggers’ lab diabetes work.
Recent publication on this project: Flood et al, 2022; Flood et al, 2020; Eggers and Carreon, 2020; Moore-Dotson and Eggers, 2019; Moore-Dotson et al, 2016
Funding for the work on diabetic retinal damage comes from the National Eye Institute and Retinal Research Foundation with past funding from JDRF and the International Retinal Research Foundation.
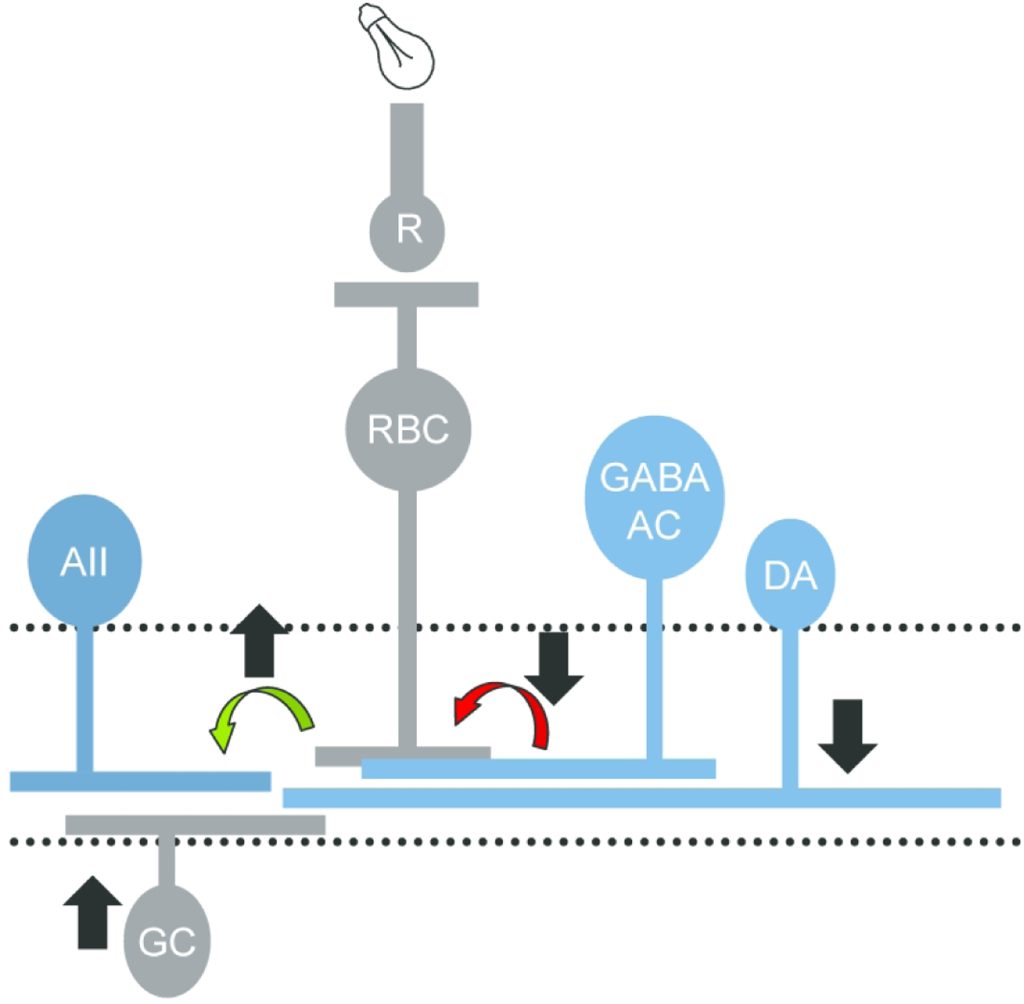
How does modulation of retinal inhibition contribute to retinal adaptation to increased light levels?
At many common light intensities, such as walking around a city at night, both rod photoreceptors that signal dim light and cone photoreceptors that signal bright light are active and carried across the same pathways toward the output of the retina. However, it is not known how these signals are combined in the retinal circuitry, particularly in the OFF pathway, which responds to the offset of a light stimulus. OFF bipolar cells, which transmit photoreceptor signals, and OFF ganglion cells, the output of the retina, are split into multiple pathways that likely signal different portions of the visual signal. We are testing the specializations of OFF retinal pathways for rod and cone light information using electrophysiological recordings of light-evoked currents. We have found that inhibitory information to OFF bipolar cells undergoes a shift from glycinergic to GABAergic input when light information shifts from rod to cone activation. The proportions of these two inhibitory inputs vary between OFF bipolar cell pathways, suggesting a specialization for rod activity. Additionally, this shift from narrow-field glycinergic inhibition to wide-field GABAergic inhibition may result in changes in visual acuity, an issue which we are actively investigating.
Recent publications on this project:
Mazade and Eggers, 2016; Eggers, Mazade and Klein, 2013; Mazade and Eggers 2013; Flood, Moore-Dotson and Eggers, 2018; Mazade, Flood and Eggers, 2019; Mazade and Eggers, 2020; Flood and Eggers, 2021.
Funding for the work on adaptation of retinal inhibition comes from the National Science Foundation and the University of Arizona Office of Research and Discovery, with past funding from the Army Research Office .
What mechanisms create the long timecourse of inhibitory neurotransmitter release in the retina?
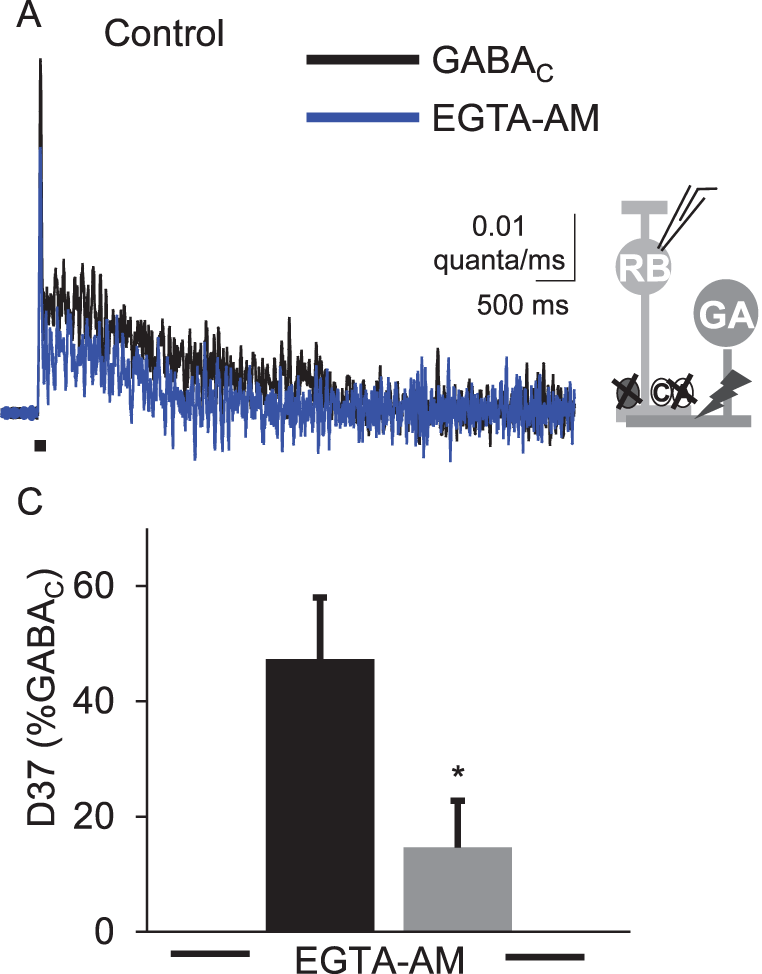
Previous results (Eggers et al, 2006) showed that the timecourse of GABA and glycine release underlying light-evoked inhibition onto bipolar cells was very slow, relative to the initial light stimulus. In other systems, prolonged, asynchronous release often results from a large build-up of Ca2+ in the presynaptic neuron. However, as the light-evoked release of GABA and glycine requires transmission through synapses upstream from the amacrine cells, it is possible that this prolonged release is not due to inherent release properties of the amacrine cells. To test this idea we are directly activating amacrine cell inputs to bipolar cells with electrical stimulation. This will allow us to test the mechanisms responsible for this slow release.
Recent publications on this project:
Eggers, Klein and Moore-Dotson, 2013; Moore-Dotson et al, 2015; Moore-Dotson and Eggers, 2019